Tuesday, October 03, 2006
DIY DNA Extraction
Precautions and safe working:
It is your responsibility to carry out a proper and sufficient risk assessment before conducting this experiment. The equipment and materials used are not particularly dangerous, but several are potentially hazardous.
Sharp: Blades.
Flammable: Ethanol.
Toxic: Ethanol - if swallowed.
What you will need:
Kiwi fruit (a ripe one works best)
Salt Solution
Washing up liquid (A supermarket own brand is perfect. The cheaper the better, as expensive brands have a lot of additives that interfere with the extraction)
Knife.
Chopping Board.
Measuring Jug.
Water Container – for beaker to sit in surrounded by water.
Beaker
Warm water (about 60oC).
Container of ice.
Ethanol
Test tube and holder
Paper clip or piece of fuse wire.
Funnel with Filter paper.
What to do:
• Put the bottle of Ethanol in the ice, to cool it down.
• In a beaker mix approx 2 grams of salt and 100mls of water or used supplied salt solution.
• Add 4 ml of washing up liquid, gently swirl to mix the contents, do not froth. Place the beaker in the bowl of warm water.
• Peel a kiwi fruit and coarsely chop it into small pieces. DO NOT reduce it to a mush. Scoop the pieces into the beaker with the water, salt and washing up liquid and gently mix the contents.
• Replace the beaker in the bowl of warm water and leave for 15 minutes.
• Filter the green mush into a test tube.
• Very carefully run the ice-cold ethanol down the inside of the test- tube onto the top of the green layer. Add about as much ethanol as there is green liquid; put the test tube into the holder and watch.
• Almost immediately you should see a fluffy white layer beginning to form at the boundary between the green and the purple liquids. This is the DNA that you have extracted from your Kiwi fruit. After a few minutes the DNA may float to the surface of the ethanol. The layer continues to grow for some time so have patience!
• It may be possible to scoop the DNA out of the liquid by using glass hook or a loop of wire, such as piece of bent fuse wire or a paper clip.
What is going on?
All the cells of the Kiwi fruit contain DNA. Slicing, chopping and blending the fruit breaks open many of the cells and allows the detergent/water/salt mixture to enter the cells and dissolve their contents, including the DNA. The salt helps dissolve the cell contents in the water, while the detergent helps breakdown the fatty membranes in the cells, making the extraction more efficient. The broken cells release three types of large molecule; the DNA; the proteins, which get eaten by the proteases in the kiwi fruit; and RNA, which is unstable and quickly falls apart in water.
The DNA molecule is very fragile so it is important not to 'blend' the fruit for too long, or shake the beaker too vigorously.
Filtering the juice removes large bits of debris.
Finally, DNA is not soluble in alcohol, so floating a layer of ice cold Ethanol over the solution of DNA causes the DNA at the interface between water and alcohol to come out of solution, where it becomes visible as fluffy white threads.
TEACHER POINTS:
- Laboratory Worksheet and Teacher Guide – including Technicians Requirements - of similar experiment is available at: http://www.ncbe.reading.ac.uk/NCBE/PROTOCOLS/plantdna.html
While this experiment demonstrates the chemistry behind DNA extraction it is not a practical method of obtaining DNA for use in further investigations. The identification of the white material as DNA is a matter of trust, which some children may find unsatisfactory.
The major value of this experiment is in demystifying DNA. “A recent study in the UK revealed that 40 percent of the population thought that only genetically engineered fruit contains DNA. It is not universally appreciated that DNA is in EVERY living thing” – Source http://www.chaosscience.org.uk/pub/public_html//article.php?story=20040206021823942 which is has another worksheet and recommended illustrated explanation of this experiment.
This experiment can easily be done in the home environment using simple kitchen equipment and common ingredients. It is recommended that using this as homework is considered for appropriate students. The “Kitchen Chemistry” version of this experiment provides three valuable outcomes.
1: Demystification of, and breaking down barriers to, science, and emphasis the ubiquity of DNA and natural biochemistry in the “real world”.
2: Simple success of apparently hard biochemistry
3: Home environment involvement in the science course
Two worksheets for this are:
- http://www.jic.ac.uk/corporate/search/print.asp?ref=http://www.jic.bbsrc.ac.uk/Corporate/media-and-public/diy-dna.htm
- http://www.york.ac.uk/res/sots/downloads/diydna.pdf
RESOURCE REQUIREMENTS:
Per Group -
Kiwi fruit (a ripe one works best)
50 g Salt (standard, pre-ground table salt works fine)
100 ml Washing up liquid (A supermarket own brand is perfect. The cheaper the better, as expensive brands have a lot of additives that interfere with the extraction)
Scalpel or knife
Chopping Board.
Measuring Cylinder
Small Container for water bath – and source of warm water (60C)
Beaker
Container of ice
500 ml Bottle of Ethanol (preferably precooled).
Test Tubes and Holders
Paper clip or piece of fuse wire.
Funnel with Filter paper.
Thursday, September 21, 2006
Quotes on questioning
The improver of natural knowledge absolutely refuses to acknowledge authority, as such. For him, scepticism is the highest of duties; blind faith the one unpardonable sin. -- Thomas H. Huxley
The important thing is not to stop questioning. Curiosity has its own reason for existing. One cannot help but be in awe when he contemplates the mysteries of eternity, of life, of the marvelous structure of reality. It is enough if one tries merely to comprehend a little of this mystery every day. Never lose a holy curiosity. -- Albert Einstein
Do not believe in anything simply because you have heard it.
Do not believe in anything simply because it is spoken and rumoured by many.
Do not believe in anything simply because it is found written in your religious books.
Do not believe in anything merely on the authority of your teachers and elders.
Do not believe in traditions because they have been handed down for many generations.
But after observation and analysis, when you find that anything agrees with reason and is conducive to the good and benefit of one and all, then accept it and live up to it. -- Gautama Buddha
Hattip Junk Science
The Disadvantages of Being Educated
I am worried by Child Centric theories of education in that the needs and wants of children, their parents and the government may be at odds with the duties of teachers to actually educate children.
As Albert Jay Nock (1937) said somewhat ironically in "The Disadvantages of Being Educated" :
My interest in education had been comfortably asleep since my late youth, when circumstances waked it up again about six years ago. I then discovered that in the meantime our educational system had changed its aim. It was no longer driving at the same thing as formerly, and no longer contemplated the same kind of product...
The difference seemed to be that while education was still spoken of as a "preparation for life," the preparation was of a kind which bore less directly on intellect and character than in former times, and more directly on proficiency. It aimed at what we used to call training rather than education; and it not only did very little with education, but seemed to assume that training was education, thus overriding a distinction that formerly was quite clear. Forty years ago a man trained to proficiency in anything was respected accordingly, but was not regarded as an educated man, or "just as good," on the strength of it. A trained mechanic, banker, dentist or man of business got all due credit for his proficiency, but his education, if he had any, lay behind that and was not confused with it. His training, in a word, bore directly upon what he could do or get, while his education bore directly on neither; it bore upon what he could become and be.
...Training is excellent, it can not be too well done, and opportunity for it can not be too cheap and abundant. Probably a glorified crèche for delayed adolescents here and there is a good thing, too; no great harm in it anyway. Yet it struck me as apparently it struck others, that there should also be a little education going on. Something should be done to mature the national resources of intellect and character as well as the resources of proficiency; and, moreover, something should be done to rehabilitate a respect for these resources as a social asset....
It had never occurred to me that there might be disadvantages in being educated. I saw at once where my mistake lay. I had been looking at the matter from the point of view of an elderly person to whom such education as he had was just so much clear gain, not from the point of view of a youth who is about to make his start in the world. I saw at once that circumstances, which had been more or less in favour of my educated contemporaries, were all dead against the educated youngster of to-day. Therefore, last year, when I was appointed to deal again with the subject in a public way, I went back on all I had said, .....
Education is divisive, separatist; training induces the exhilarating sense that one is doing with others what others do and thinking the thoughts that others think.
Education, in a word, leads a person on to ask a great deal more from life than life, as at present organized, is willing to give him; and it begets dissatisfaction with the rewards that life holds out. Training tends to satisfy him with very moderate and simple returns. A good income, a home and family, the usual run of comforts and conveniences, diversions addressed only to the competitive or sporting spirit or else to raw sensation - training not only makes directly for getting these, but also for an inert and comfortable contentment with them. Well, these are all that our present society has to offer, so it is undeniably the best thing all round to keep people satisfied with them, which training does, and not to inject a subversive influence, like education, into this easy complacency. Politicians understand this - it is their business to understand it - and hence they hold up "a chicken in every pot and two cars in every garage" as a satisfying social ideal. But the mischief of education is its exorbitance. The educated lad may like stewed chicken and motor-cars as well as anybody, but his education has bred a liking for other things too, things that the society around him does not care for and will not countenance. It has bred tastes which society resents as culpably luxurious, and will not connive at gratifying. Paraphrasing the old saying, education sends him out to shift for himself with a champagne appetite amidst a gin-guzzling society.
Training, on the other hand, breeds no such tastes; it keeps him so well content with synthetic gin that a mention of champagne merely causes him to make a wry face. ...
...the educated youth starts under disadvantages from which the trained youth is free. The trained youth has no incentive to regard these matters except as one or another of them may bear upon his immediate personal interest. Again, while education does not make a gentleman, it tends to inculcate certain partialities and repugnances which training does not tend to inculcate, and which are often embarrassing and retarding. They set up a sense of self-respect and dignity as an arbiter of conduct, with a jurisdiction far outreaching that of law and morals; and this is most disadvantageous. Formerly this disadvantage was not so pressing, but now it is of grave weight....
At the present time, as we have lately been reminded, the exigencies of politics have converted candidacy for public office into an exact synonym for an obscene and repulsive exhibitionism.
Again, education tends towards a certain reluctance about pushing oneself forward; and in a society so notoriously based on the principle of each man for himself, this is a disadvantage.....
Things may change for the better, in time; no doubt they will. Economic opportunity may, by some means unforeseen at present, be released from the hold of its present close monopoly. The social value of intellect and character may some day be rediscovered, and the means of their development may be rehabilitated.
I'm with Nock on this educational theory - "training" for exams, jobs and citizenship is a fine thing but if I fail to ignite the spark of enquiry, independence and interest that the hallmarks of an educated mind then I will have failed. I'm pleased that my School places such importance on producing well rounded people that I'm enjoying discovering how they instil the love of learning and the encouragement of becoming "educated".
Because, if you will excuse more quotes in an over-long post, as an even more historical figure said:
"I know no safe depositary of the ultimate powers of the society but the people themselves; and if we think them not enlightened enough to exercise their control with a wholesome discretion, the remedy is not to take it from them, but to inform their discretion by education. This is the true corrective of abuses of constitutional power." --Thomas Jefferson to William C. Jarvis, 1820.
"The main objects of all science [are] the freedom and happiness of man." --Thomas Jefferson to Thaddeus Kosciusko, 1810.
Wednesday, September 13, 2006
Question 2 - CSTE - useful or not?
The advantages of representations of complex situations by graphical means often outweigh the loss of the subtle nuances. A broad brush approach to the roles of teachers can split them into four areas:
1, caring and control;
2, skills, schooling and discipline;
3, teaching and training;
4, educating and inspiring.
Individual pupils have different needs of these roles, which change as they progress through their school career. Individual lessons and schemes of work provide for these roles in differing amounts. These theoretical graphs provide pictures of what may be happening. By using the same simplified approach on these different aspects of schooling it is hoped easy comparisons of how well matched they are, could be made.

Is this approach workable, helpful or useful?
Question 1
How to motivate children to learn and to help themselves self-motivate is commonly presented in teacher training books. What is not clear is what the incentives are the children are being motivated to reach. To motivate an incentive is needed, because the logical response to the exhortation “Work harder” is “why?” As economists always say “Incentives Matter”. In business the failure to provide clear incentives is probably the commonest cause of poor motivation. Is the same true in the classroom?
Incentives can range from the abstract to the concrete and from the negative to the positive. Incentives range across a feeling of self-worth and satisfaction of curiosity, monetary rewards, peer approval, parental approval, long-term advantage, avoiding punishment etc.
For incentives to work, they must be believable and achievable. How do teachers choose which incentives to highlight and motivate pupils towards and also, how do they encourage pupils to self motivate towards the incentives?
To investigate this question would require a review of relevant literature, current practices and if possible questionnaires and interviews with pupils and teachers.
The Victorian view of motivation in school and life is summed up in
“Vitaï Lampada” by Sir Henry Newbolt
And it's not for the sake of the ribboned coat,
Or the selfish hope of a season's fame,
But his Captain's hand on his shoulder smote -
'Play up ! play up ! and play the game !'
The hundred years later, does this ethos have any relevance?
Tuesday, September 12, 2006
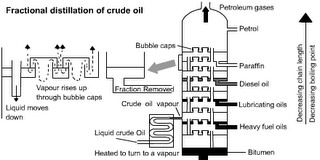
Distillation of Crude Oil
KEY STAGE 4 SC3 MATERIALS AND THEIR PROPERTIES Changing materials
2a) how the mixture of substances in crude oil, most of which are hydrocarbons, can be separated by fractional distillation
b) the use of some of the products from crude oil distillation as fuels -
Crude Oil is a MIXTURE of different compounds, in this case HYDROCARBONS (Compounds that contain Hydrogen and Carbon only) – Ensure you can explain the difference between Mixtures and Compounds. Mixtures can be separated by PHYSICAL methods such as filtration, distillation. The Hydrocarbons in Crude Oil are the important fuels and oil based products that our way of life depends on. For a virtual tour of an Oil refinery and its role see: http://www.schoolscience.co.uk/content/4/chemistry/petroleum/fawley/index.html
Crude Oil is separated by Fractional Distillation. Different Hydrocarbons have different BOILING POINTS. This means the different FRACTIONS can be separated by either heating the liquid to different temperatures and collecting the different products as they boil off or by collecting liquids as the CONDENSE at different temperatures.
The different fractions are put to different uses. Note that the fractions with the lower boiling points are thinner, lighter in colour and more flammable. They tend to be smaller molecules: For details see: http://www.schoolscience.co.uk/content/4/chemistry/petroleum/knowl/4/2index.htm?fractions.html
Hydrocarbons are split into ALKANES and ALKENES. Alkanes are said to be SATURATED because all the carbon atoms in the “spine” of the molecule are joined by single COVALENT bonds. Alkenes (Hint to remember: note the extra lines in an E versus an A) are have double bonds.
Alkane R–CH2–CH2–R CnH2n+2
Alkene R–CH=CH–R CnH2n
Source: http://www.cem.msu.edu/~reusch/VirtualText/nomen1.htm
Industrial scale fractional distillation
This uses condensation at different temperatures.
Source: http://lgfl.skoool.co.uk/examcentre.aspx?id=182
Larger molecules can be CRACKED into smaller more useful ones by the use of heating and catalysts.
Fractional Distillation should be a classroom experiment:
It can be simply done by heating a test tube and collection the distillate in a cooled receiver, taking note of the boiling temperature of each fraction or by the use of more complicated and professional apparatus.


Full details of a safer alternative to using Crude Oil see the CLEAPSS hazcard
An excellent presentation for classroom use is at:
http://lgfl.skoool.co.uk/content/keystage4/chemistry/pc/lessons/uk_ks4_oil_products/h-index.htm
Tuesday, July 18, 2006
CO2 - a minor role?
CO2 Science: "In light of these real-world-based observations, plus the multitude of studies that indicate most climate changes of the past were clearly associated with changes in solar activity (see Solar Effects in our Subject Index), the case for anthropogenic CO2 emissions playing anything more than a minor role in contemporary global warming would appear to be fading fast."